ChemInform Abstract: Synthesis and Biological Activity of Novel Thyroid Hormone Analogues: 5′-Aryl Substituted GC-1 Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
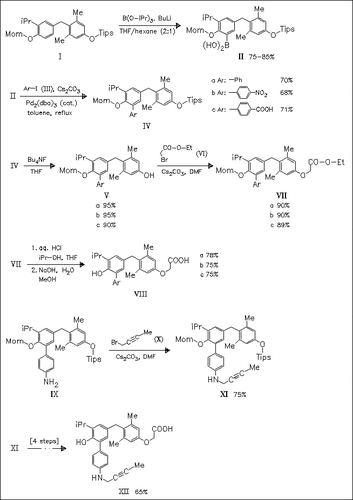
Eleven 5′-substituted GC-1 analogues like (VIII) and (XII) are synthesized to explore the effect of GC-1 core structure modifications on binding to thyroid hormone receptor isoforms and activation of transcription. This approach is based on ortho lithiation and in-situ boration of the biarylmethane (I), a key intermediate of the revised GC-1 synthesis, followed by Suzuki cross-coupling. All of the new compounds show reduced binding affinity, but most of the compounds retain TRβ-selectivity. All compounds are nearly full agonists of TRβ activation, except (VIIIb), which antagonizes the response to thyroid hormone.