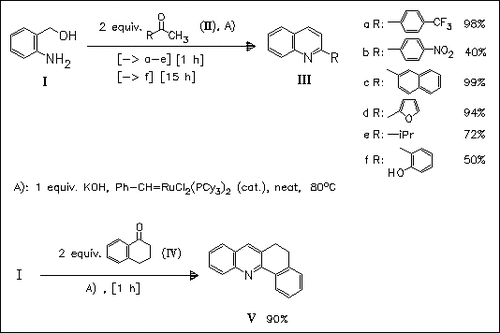
ChemInform Abstract: Ruthenium-Catalyzed Oxidative Cyclization of 2-Aminobenzyl Alcohol with Ketones: Modified Friedlaender Quinoline Synthesis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
A modified Friedlaender quinoline synthesis via a ruthenium-catalyzed oxidative cyclization of 2-aminobenzylalcohol (I) with ketones (II) is reported. A number of alkyl- and aryl ketones are readily cyclized with (I) affording the corresponding quinolines in good to excellent yields.