ChemInform Abstract: Novel Reversion Reaction of D-arabino-Hexose Phenylosotriazole. A Useful Model in Natural Glycoside and Polysaccharide Analysis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
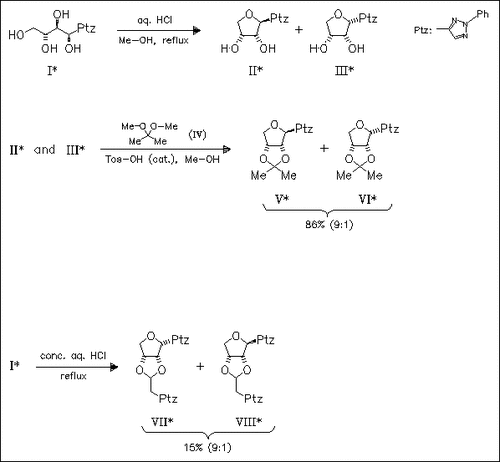
On treatment of title compound (I) with methanol/mineral acid, the formation of D-ribo and D-arabino cyclized products (II) and (III) is observed. The yield of these compounds is strongly affected by the concentration of acid. To determine the ratio of α- and β-C-glycosides, the mixture of (II) and (III) is masked as acetonides (V) and (VI). Reaction of (I) with conc. HCl in the absence of methanol leads to a new type of reversion products which consists of a mixture of two stereoisomers (VII) and (VIII). The formation of (VII) and (VIII) proceeds via reaction of anhydro derivatives (II) and (III) with in situ formed formylmethyltriazole.