ChemInform Abstract: Cleavage of the N(1)—C(4) Bond of 4-(4′-Hydroxyphenyl)-azetidine-2-ones via Quinone Methide Intermediates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
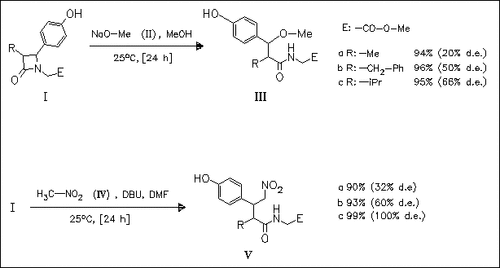
On treatment with base, azetidinones (I) undergo a novel ring-opening reaction to give quinone methide intermediates. Their 1,6-conjugate addition reactions yield the esters (III) and (V).