ChemInform Abstract: New Design Concepts for Constraining Glycosylated Amino Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
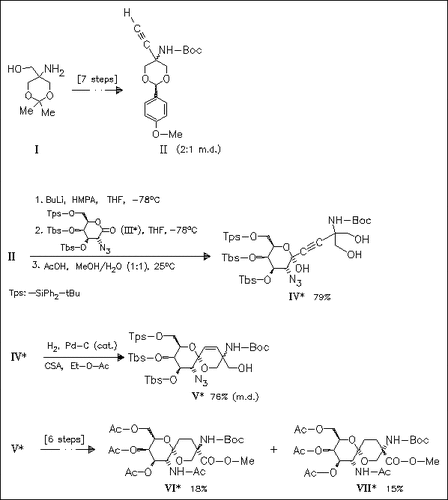
Glycopeptides (VI) and (VII), representing the D- and L-serine configuration, are prepared through a convergent strategy involving the nucleophilic addition of alkyne (II) to the lactone (III) followed by deprotection to afford diol (IV). A one-pot partial reduction/spiroketalization reaction of diol (V) gives a product mixture containing at least two epimeric spiro compounds (V). Further transformations of this mixture afford the desired separable glycopeptides.