ChemInform Abstract: Synthesis and Antiproliferative Activity of Epoxy and Bromo Compounds Derived from Estrone.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
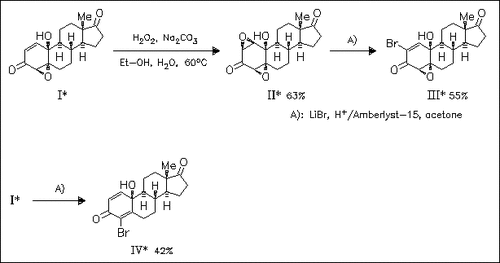
Steroidal epoxy- and bromo-compounds (II), (III), and (IV) are synthesized stereoselectively in moderate to good yields from epoxyquinol (I) using mild reaction conditions. Significant increase in the antiproliferative activity is observed by introduction of a further oxirane ring and a bromine atom into the A-ring. Cytotoxicity tests reveal that all compounds show a low selectivity between neoplastic and normal cells.