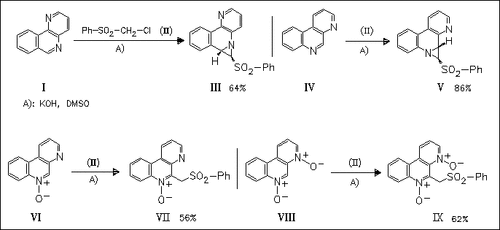
ChemInform Abstract: Vicarious Nucleophilic Substitution of Hydrogen and Formation of Aziridine Rings in Reactions of Benzonaphthyridines and Their N-Oxides with Chloromethyl Phenyl Sulfone.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Treatment of benzonaphthyridines (I) with chloromethyl sulfone (II) leads to the formation of aziridine rings anellated to the benzonaphthyridine skeleton, whereas their N-oxides undergo vicarious nucleophilic substitution of hydrogen, thus leading to the corresponding phenylsulfonylmethyl derivatives.