ChemInform Abstract: Desymmetrization Reactions: A Convenient Synthesis of Aromatic Diamide Diamines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
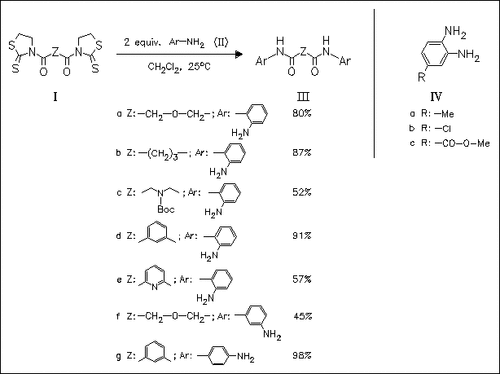
The synthesis of various diamine diamides (III) derived from diaminobenzenes is described, involving their reaction with bis(thiazolidinethiones) (I) under mild reaction conditions. When substituted diaminobenzene derivatives like (IV) are used, the amidation reactions proceed with exclusive regioselectivity (product structure is not given). 2,3-Diaminonaphthalene does not undergo amidation reaction, probably due to its low nucleophilicity.