ChemInform Abstract: Regioselective Superacid-Catalyzed Electrocyclization of Diphenylmethyl Cations to Fluorenes, Phenanthrols and Benzofurans.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
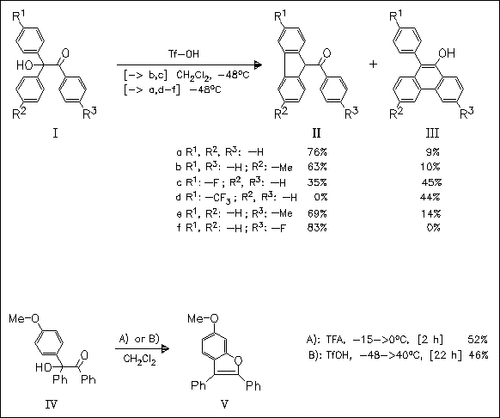
Electrocyclization of in situ generated diphenyl(benzoyl)methane cations in the presence of superacid TfOH provides benzofuran (V), fluorene (II) or phenanthrene derivatives (III) depending on the substitution pattern at the aromatic rings. Additionally, the nature of substituents at the diarylmethyl rings determines the regioselectivity of cyclization [cf. (IIIb) vs. (IIIc)].