ChemInform Abstract: Stereoselective Synthesis of Dihydropyrone-Containing Marine Natural Products. Total Synthesis and Structural Elucidation of (-)-Membrenone-C.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
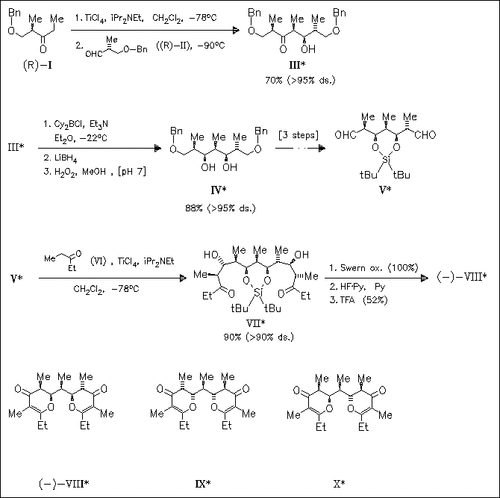
The total synthesis of three diastereomers of membrenone C (VIII)—(X) on similar ways is presented. Key steps are the diastereoselective aldol coupling of ketone (I), the stereoselective reduction of the carbonyl group in aldol adduct (III), and the titanium-mediated two direction aldol coupling to introduce the remaining chain carbons into dialdehyde (V). Comparison of NMR spectra and optical rotation of the three diastereomers (VIII)—(X) with a sample of naturally occurring (-)-membrenone C reveals membrenone C to have the structure of compound (VIII).