ChemInform Abstract: Preparation of 2,2′-Dihydroxytriphenylmethanes Using Metal Phenolates with Aromatic Aldehydes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
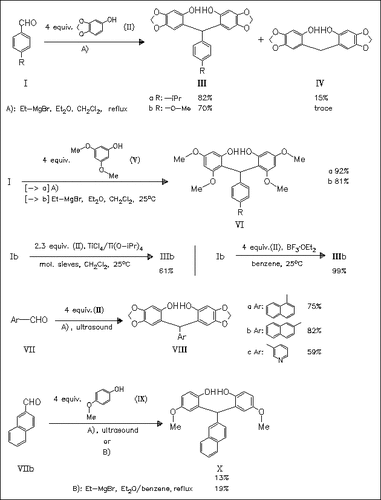
Bisarylation of aromatic aldehydes bearing electron-donating substituents [e.g. (I)] by phenolates is suitable for electron-rich phenols and provides a regiospecific route to 2,2′-dihydroxytriphenylmethanes such as (III) and (VI). This arylation procedure is extended to naphthaldehydes and pyridine carboxaldehydes to prepare bisphenols like (VIII), but in low yield. However, sonication leads to better yields. Reaction of naphthaldehyde (VIIb) with methoxyphenolate (IX) in dichloromethane fails, but replacement of the solvent or sonication gives the desired bisarylation product (X) in low yield.