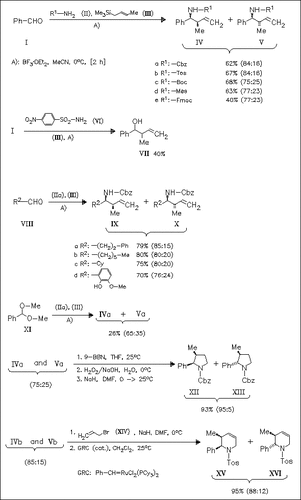
ChemInform Abstract: Syn Diastereoselectivity in the Synthesis of Homoallylamine Using Crotylsilane in the Three-Component Reaction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The use of crotylsilane (II) in the Lewis acid catalyzed three-component reaction with aldehydes and carbamates gives generally homoallylamines with good syn selectivity under the optimized conditions A). The products can be transformed into pyrrolidines and piperidines.