ChemInform Abstract: Contributions on Crystal Structures and Thermal Behavior of Anhydrous Phosphates. Part 29. Preparation and Structure Determination of SrMn2(PO4)2 and Redetermination of β′-Mn3(PO4)2.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Single crystals of SrMn2(PO4)2 are obtained from a mixture of SrCl2×6H2O, Mn(OAc)2, and NH4H2PO4 (1. 873 K, 6 h; 2. 1173 K, 24 h; 3. 1373 K). The compound crystallizes in the triclinic space group P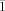 with Z = 4 and represents a new structure type characterized by a hexagonal close packing of phosphate groups. In addition, single crystals of β′-Mn3(PO4)2 are grown by chemical vapor transport using a phosphorus/iodine mixture as transport agent (850→800 °C). The reinvestigation of its structure (monoclinic, space group P21/c, Z = 12) yields improved R-values and significantly reduced standard deviations for the interatomic distances.
with Z = 4 and represents a new structure type characterized by a hexagonal close packing of phosphate groups. In addition, single crystals of β′-Mn3(PO4)2 are grown by chemical vapor transport using a phosphorus/iodine mixture as transport agent (850→800 °C). The reinvestigation of its structure (monoclinic, space group P21/c, Z = 12) yields improved R-values and significantly reduced standard deviations for the interatomic distances.




