ChemInform Abstract: Synthetic Studies on Brevetoxin-B. Part 3. Stereoselective Synthesis of the IJK-Ring System.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
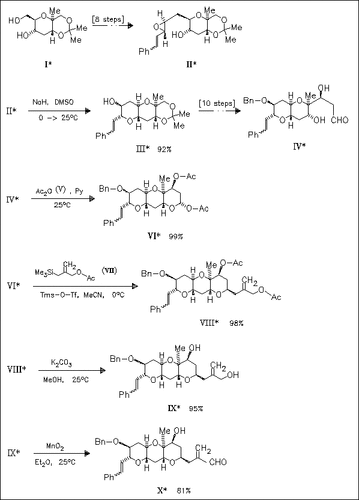
The synthesis of title compound (X) starts from the J-ring compound (I). Further key features are the formation of the I-ring by 6-endo cyclization of the hydroxy styrylepoxide (II) and the direct introduction of the C-4-unit (VII) as the side chain.