ChemInform Abstract: Synthetic Studies on Brevetoxin-B. Part 2. Stereoselective Synthesis of the EFG-Ring System.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
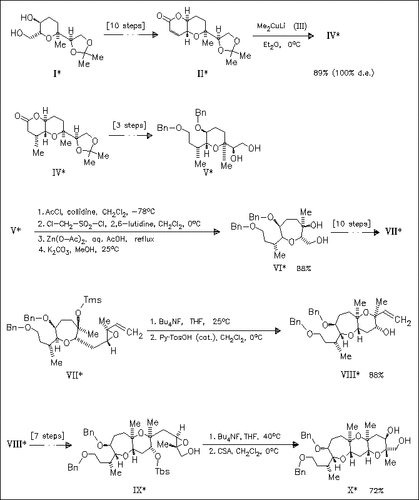
The title compound (X) is synthesized via stepwise construction of the E, F, and G ring using a stereoselective Michael addition of the α-methyl group, a novel ring expansion of compound (V), and 6-endo cyclizations of vinylepoxide (VII) and methylepoxide (IX).