ChemInform Abstract: Synthesis of the Bisbenzanellated Spiroketal Core of the γ-Rubromycins. The Use of a Novel Nef-Type Reaction Mediated by Pearlman′s Catalyst.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
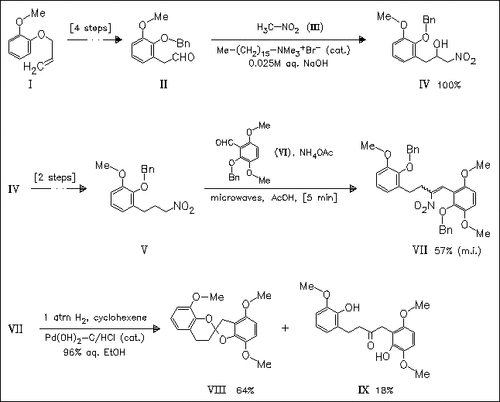
The first synthesis of the bisbenzanellated spiro system (VIII) is presented using a novel Nef-type reaction of conjugated nitroalkene (VII) mediated by Pearlman′s catalyst. Precursor (VII) is prepared from readily synthesized starting materials using Henry condensations of aldehydes with nitroalkanes.