ChemInform Abstract: Oxazolium-Derived Azomethine Ylides. External Oxazole Activation and Internal Dipole Trapping in the Synthesis of an Aziridinomitosene.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
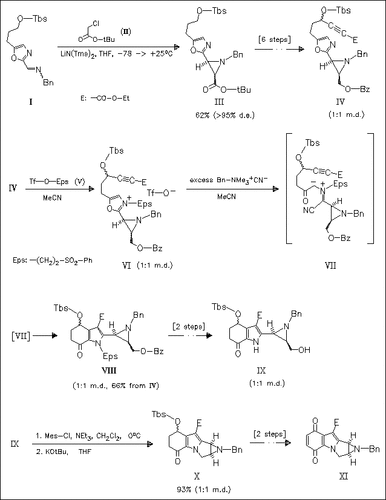
It is shown that benzylated aziridine derivatives of type (IV) can be selectively alkylated at the oxazole nitrogen yielding salts like (VI). Treatment of the latter with Bn-NMe3CN gives azomethine ylides [see (VII)] which undergo cycloaddition forming indoles like (VIII). Interestingly, under these conditions the aziridine ring remains intact. (VIII) can smoothly be transformed into the aziridinomitosene derivative (XI).