ChemInform Abstract: An Easy Entry to (1S,2S) and (1R,2R)-threo-Ifenprodil.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
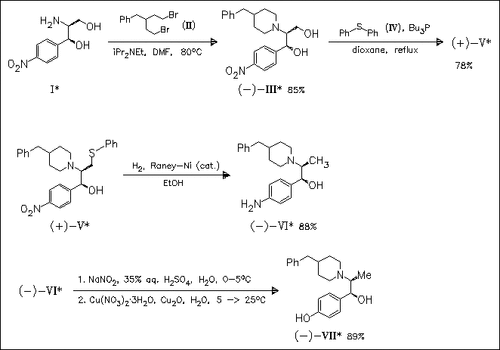
A facile and practical synthesis of enantiomerically pure L- and D-threo-ifenprodil [cf. (VII)], key intermediates in the synthesis of amphenicol-type antibiotics, starting from corresponding enantiopure threo-2-aminopropanediols (I) is accomplished.