ChemInform Abstract: Synthesis and Biological Evaluation of L- and D-Configuration 1,3-Dioxolane 5-Azacytosine and 6-Azathymine Nucleosides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
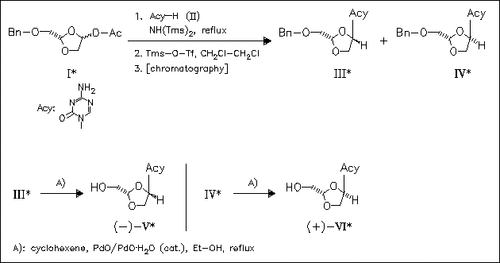
The effects of combining the structural feature of the oxygen containing sugar of (-)-L-β-1,3-dioxolanyl cytosine with various bases of active azanucleosides are investigated. A novel class of dioxolane azapyrimidine nucleoside analogues is synthesized. Azacytosine derivative (V), having L-configuration, shows significant activity against HBV, whilst the D-configuration analogue reveals potent anti-HIV activity. The 6-azathymine analogues display no activity.