ChemInform Abstract: Synthesis of 3,7-Anhydro-D-glycero-D-ido-octitol 1,5,6-Trisphosphate as an IP3 Receptor Ligand Using a Radical Cyclization Reaction with a Vinylsilyl Tether as the Key Step. Conformational Restriction Strategy Using Steric Repulsion Between Adjacent Bulky Protecting Groups on a Pyranose Ring.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
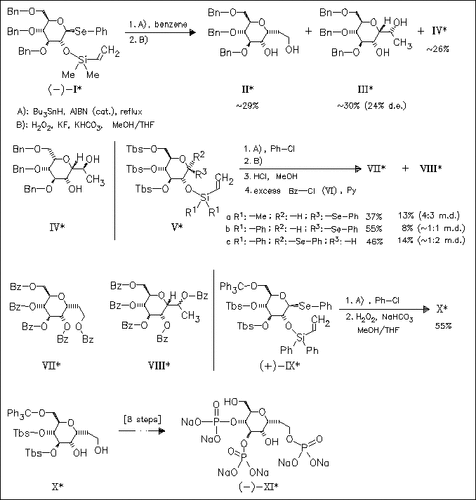
The title phosphate (XI), a potent IP3 receptor ligand, is prepared successfully via thermodynamically controlled radical cyclization of the vinylsilyl ether (IX) followed by oxidative ring opening yielding the α-C-glycoside (X). The outcome of this key step is found to depend on conformational effects. Thus, an unusual 1C4-conformation (due to steric repulsion between bulky silyl protecting groups) leads to preferred formation of desired α-C-glycosides.