ChemInform Abstract: [2 + 2] Cycloaddition of Chlorosulfonyl Isocyanate to Allenylsugar Ethers.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
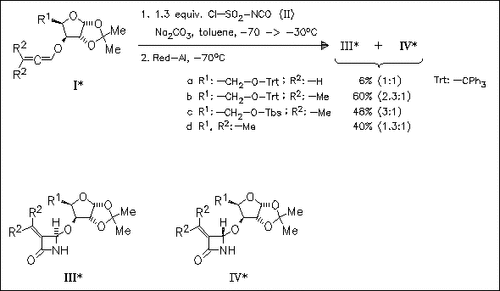
The [2 + 2] cycloaddition of allenes and chlorosulfonyl isocyanate provides β-lactams with a moderate stereoselectivity. A stereochemical model of the transition state of the cycloaddition is proposed, based on the lowest energy conformation of the cumulene.