ChemInform Abstract: Rearrangement Reactions in the Fluorination of 3-Deoxy-3-C-methyl-3-nitro-hexopyranosides (and Hexo-1-thiopyranosides) of the D- and L-Series by the DAST Reagent.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
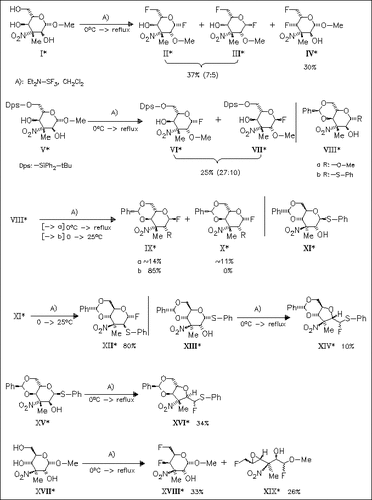
A detailed study on the recently briefly investigated title reaction is presented. Three types of rearrangement mechanism are observed depending on the 1,2-stereochemical pattern of the substrate. In case of 1,2-cis configured substrates, the outcome of the reaction depends on presence or absence of an C-4 hydroxyl protecting group.