ChemInform Abstract: Syntheses and Properties of Silicon- and Germanium-Containing α-Amino Acids and Peptides: A Study on C/Si/Ge Bioisosterism.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
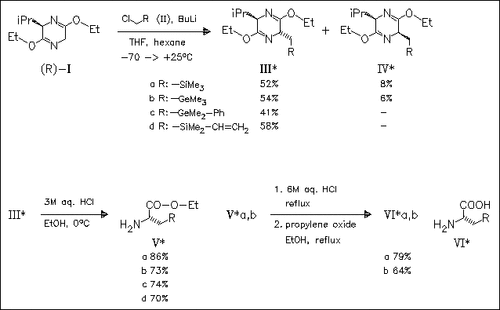
Synthesis of silicon- and germanium-containing α-amino acids (VI) and (analogues of β-tert-butylalanine) is achieved starting with the diastereoselective alkylation (60-74% d.e.) of optically active dihydropyrazine (I) with the corresponding silyl- or germylmethyl chlorides (II) to provide, after liquid chromatography, substituted dihydropyrazines (III) and (IV) in excellent diastereomeric purity. Acids (VI) and a carba-analogue are incorporated into position 5 of a decapeptide (analogue of the potent GnRH antagonist Cetrorelix). Evaluation of the biological activity of these decapeptides shows that they all are potent GnRH antagonists, but silicon- and germanium-containing peptides show an advantage (longer duration of biological activity) over the carba-analogue.