ChemInform Abstract: Synthesis of (+)- and (-)-Ferruginol via Asymmetric Cyclization of a Polyene.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
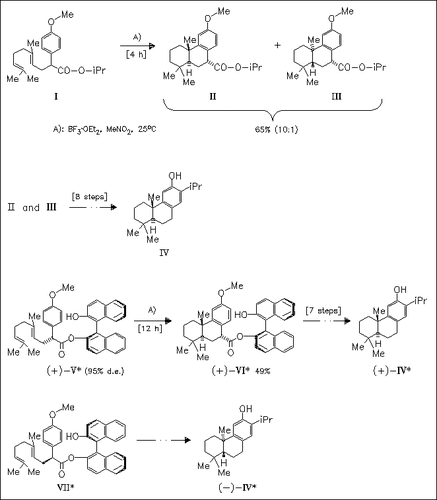
Ferruginol (IV) is synthesized via a route involving the treatment of esters (I), (V), or (VII) with BF3×OEt2 to give either racemic or pure enantiomeric compound (IV), which by further elaboration is transformed to the target terpene.