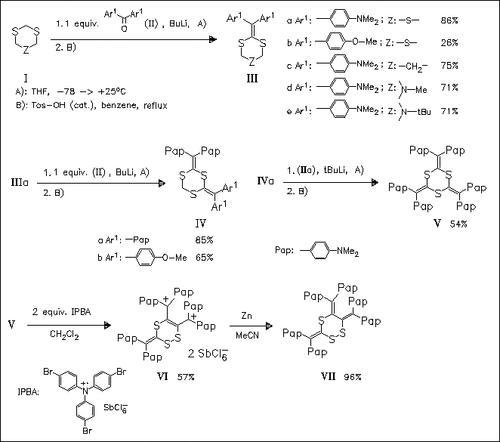
ChemInform Abstract: Preparation, Structure, and Unique Redox Properties of Mono-, Bis-, and Tris(diarylmethylene)-1,3,5-trithianes and Related Compounds.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
A number of title compounds like (III)—(V) are prepared. Voltammetric analysis shows that a large structural change and/or transannular bonding are induced during their electrochemical oxidation. Bis- and tris(diarylmethylene)-1,3,5-trithianes undergo easy skeletal rearrangement to 1,2,4-trithiane ring systems upon oxidation.