ChemInform Abstract: Synthesis and Reactions of Some 2-Vinyl-3H-quinazolin-4-ones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
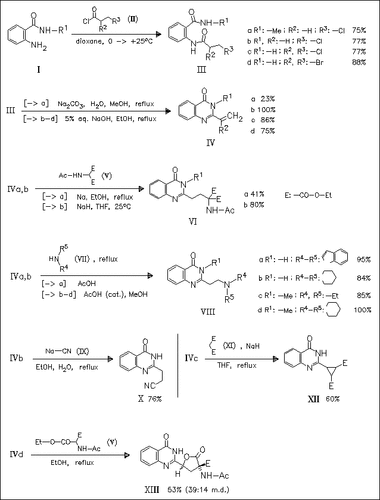
Synthesis of title vinylquinazolinones (IV) is smoothly achieved by base-catalyzed cyclization of the corresponding o-(ω-halopropionyl)aminobenzoic acid amides (III). Compounds (IVa) and (IVb) are good Michael acceptors that undergo reactions with various C- or N-nucleophiles at the vinyl group to furnish the corresponding adducts, e.g. (VI), (VIII), and (X). Halo-substituted vinylquinazolinones (IVc) and (IVd) are also good Michael acceptors, but due to secondary reactions like dehydrohalogenation or cyclization other products are obtained, e.g. cyclopropane (XII) or furan derivatives like (XIII).