ChemInform Abstract: Synthesis of Benzo[f]quinoline Derivatives by Condensation of Arylmethylene-2-naphthylamines with Ethyl Acetoacetate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
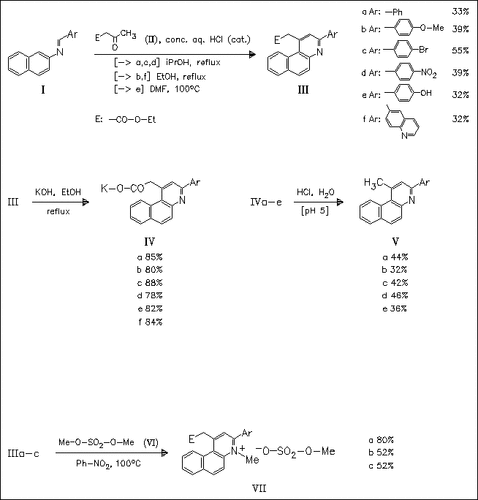
Condensation of title arylmethylenenaphthylamines (I) with acetoacetate (II) proceeds smoothly in the presence of catalytic amounts of HCl to give ethoxycarbonylmethyl-substituted benzo[f]quinolines (III) in moderate yields. The corresponding salts (IV) undergo decarboxylation during acidification with mineral acids. The N-methylbenzoquinolinium salts (VII), potential novel antiviral agents, are obtained on N-methylation with dimethyl sulfate. All compounds prepared are characterized by means of NMR spectroscopy.