ChemInform Abstract: Reaction of Pyrrole Anions with Carbon Disulfide. Synthesis of Pyrrole-3-carbodithioates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
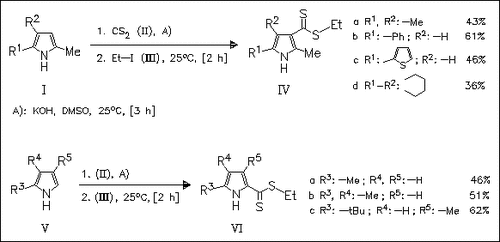
Treatment of 2,5-disubstituted pyrroles (I) with carbon disulfide and KOH/DMSO followed by ethyl iodide affords a direct synthesis of 3-pyrrole-carbodithioates (IV). 2-Methyl-, 2,3-dimethyl- and 2,4-dialkylpyrroles (V) give exclusively 2-carbodithioates (V) under the same conditions. The results are in complete contrast to unsubstituted pyrrole which selectively form the 1-carbodithioates.