ChemInform Abstract: Donor/Acceptor Fulleropyrrolidine Triads.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
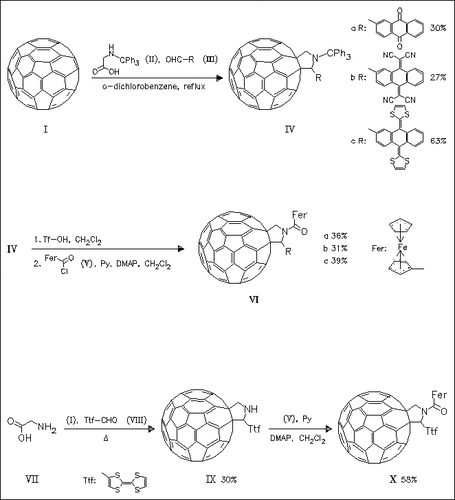
In the novel title compounds (VI) and (X) a noticeable electronic interaction between the electroactive units is observed. Interestingly, (VIa) and (VIb) show reduction potentials similar to that of C60, whereas (VIc) and (X) show reduction potentials which are cathodically shifted in comparison to parent C60. In (VIc) and (X) intramolecular electron transfer processes occur from TTF or extended TTF to the fullerene singlet excited state, rather than from the ferrocene donor.