ChemInform Abstract: Electrochemical Oxidative Coupling of 1,4-Bis(1,4-dithiafulven-6-yl)benzene Cyclophanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
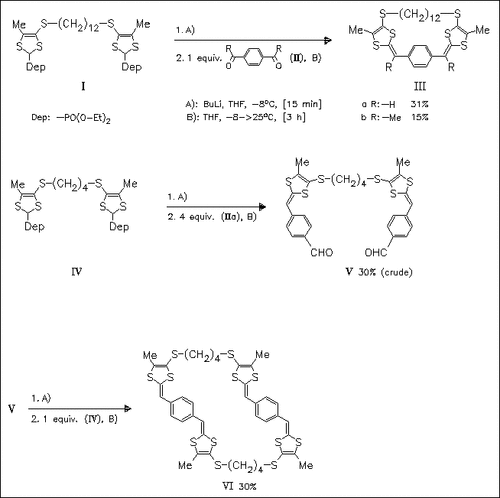
Novel bridged bis(dithiafulvenyl)benzenes are synthesized by Wittig—Horner olefinations and the redox behavior of these compounds is studied by cyclic voltammetry. Cyclophanes (IIIa) and (VI) are found to undergo electrochemical oxidative coupling to give cyclooligomers.