ChemInform Abstract: Highly Efficient and Selective Electrophilic and Free Radical Catalytic Bromination Reactions of Simple Aromatic Compounds in the Presence of Reusable Zeolites.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
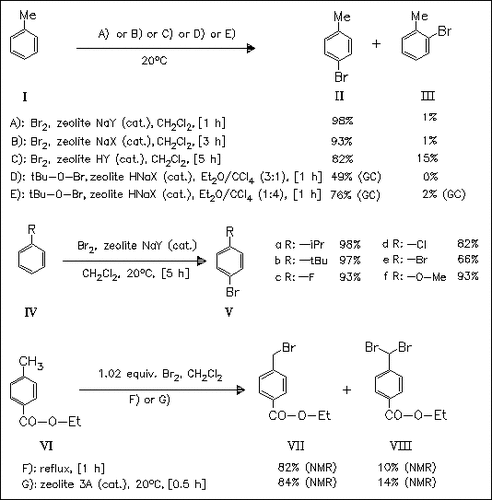
The reaction of bromine with substituted benzenes of moderate activity in the presence of sufficient NaY zeolite at room temperature provides a convenient, high yielding and highly regioselective method for the synthesis of para-brominated products. It is found, that heating easily regenerates the zeolite and the reaction works almost as well on a vastly larger scale, thus representing a commercially realistic method for the synthesis of p-bromo-substituted benzenes. High para-selectivity is also achieved in the bromination of toluene with tBuOBr in the presence of HNaX zeolite as catalyst, but the reaction is not general. The use of various zeolites (3A, 4A, 5A, 13X, NaM, HY and Hβ) in the light-induced side-chain bromination of benzoate (VI) enhances the reaction rate, but does not substantially diminish the tendency to over-bromination.