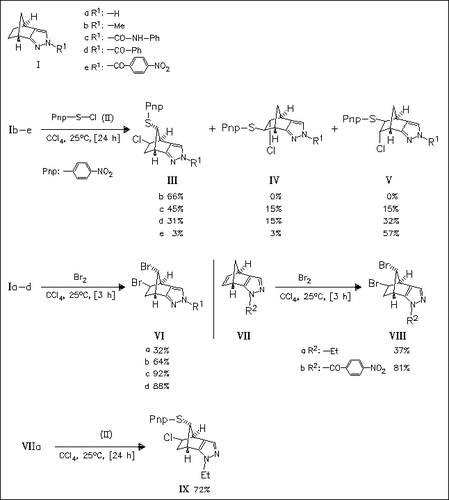
ChemInform Abstract: Neighboring Effect of Pyrazole Rings: Regio- and Stereoselective Wagner—Meerwein Rearrangement in Electrophilic Addition Reactions of Norbornadiene-Fused Pyrazoles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Fused pyrazoles (I) and (VII) undergo electrophilic addition of sulfenyl chloride (II) or bromine to afford skeletally rearranged adducts in a regio- and stereoselective manner which is attributable to the neighboring group participation of the pyrazole ring accompanied by the regioselective formation of a bridged pyrazolium ion.