ChemInform Abstract: Synthesis and Properties of Stable Heteroazulene Analogues of a Triphenylmethyl Cation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
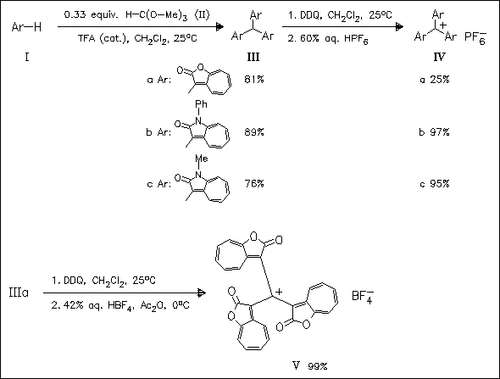
The route to title compounds (IV) and (V) involves TFA-catalyzed electrophilic aromatic substitution of heteroazulenes (I) with orthoformate (II), followed by oxidative hydrogen abstraction with DDQ and subsequent exchange of the counter-anion by using aq. HPF6 or aq. HBF4. The spectrophotometrically determined pKR+-values are in the range of 9.7—13.1. A good correlation between the pKR+-values and the reduction potentials, determined by cyclic voltammetry, are obtained for the title cations.