ChemInform Abstract: Asymmetric Cyclopropanation of Styrene Catalyzed by Cu—(Chiral Schiff-Base) Complexes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
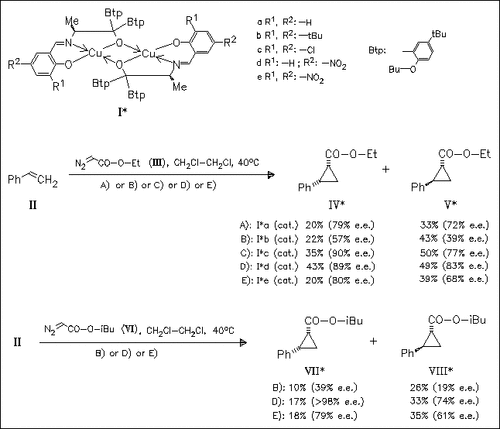
Various novel copper—chiral Schiff base complexes are prepared and applied as catalysts in the cyclopropanation reaction of styrene (II) with diazoacetates. The influence of the salicylidene ring substitution pattern of the Schiff bases on the enantioselectivity of cyclopropanation reaction is studied. It is shown that electron-withdrawing substituents [cf. (Ic) and (Id)] enhance the enantioselectivity, but bulky substituents in ortho position decrease the selectivity [cf. (Ie)]. In general, optical purity of the cis-adducts is higher than that of the trans-adducts.