ChemInform Abstract: Reactions of Alkyl Azides and Ketones as Mediated by Lewis Acids: Schmidt and Mannich Reactions Using Azide Precursors.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
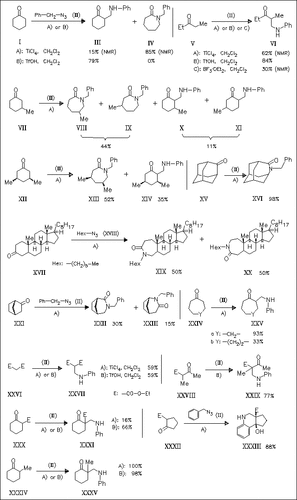
Reactions of ketones with alkyl azides in the presence of either TiCl4 or TfOH are investigated in continuation of previously published studies. In general, simple cyclic ketones with 3, 4 and 6 ring members undergo Schmidt reactions with benzyl azide in the presence of TiCl4 while cyclic ketones with 5 or more than 6 members as well as acyclic ketones undergo azido-Mannich reactions after rearrangement of the benzyl azide. These Mannich-type reactions are most efficiently mediated by TfOH. Some bridged systems prefer the Schmidt pathway. The results are discussed based on steric and conformational considerations.