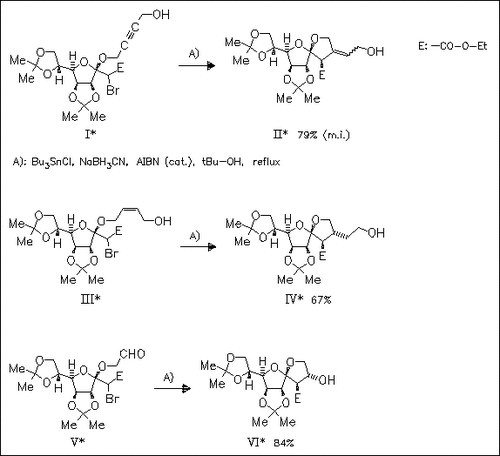
ChemInform Abstract: Radical Mediated Stereoselective Synthesis of Chiral Spiroacetals from Enol-Esters.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The regio- and stereoselective radical cyclization of chiral α-bromo acetals gives highly functionalized spiroacetals with a variety of functional groups.