ChemInform Abstract: Stereocontrolled Synthesis of a Novel Pharmacophore of the Tubulin-Depolymerizing Marine Natural Product Spongistatin.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
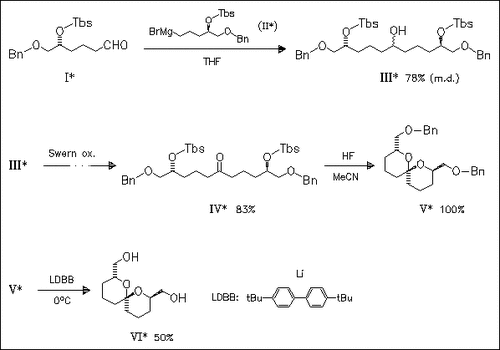
The stereoselective synthesis of (VI) is achieved in 11 steps starting from commercially available benzyl (R)-glycidyl ether. Key step of the synthesis is the high yielding acid-catalyzed intramolecular acetal formation to give (V). Compound (VI) causes tubulin-depolymerization and exhibits potent cytotoxicity against taxol-resistant and vincristine-resistant human cancer cells.