ChemInform Abstract: Total Syntheses of (.+-.)-Anchinopeptolide D and (.+-.)-Cycloanchinopeptolide D.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
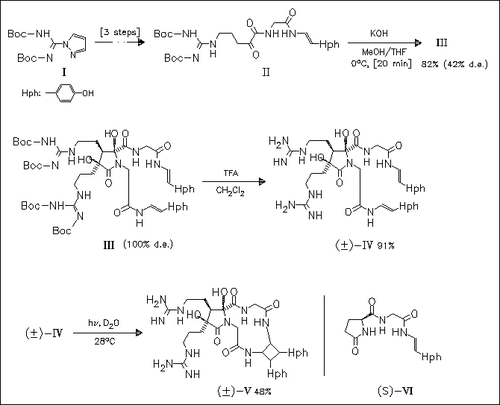
The synthesis of anchinopeptolide D (IV) is achieved via stereoselective dimerization of the keto amide (II). On irradiation in water (IV) undergoes [2 + 2] cycloaddition to afford cycloanchinopeptolide D (V). The cyclobutane ring is formed by an unprecedented head-to-head dimerization of the hydroxystyrylamide units. Additionally, it is found that naturally occurring glutamyl-N-[2-(4-hydroxyphenyl)ethenyl]glycinamide adopts the structure (VI) and not an acyclic structure as previously reported.