ChemInform Abstract: Stereoselective Carbon—Carbon Bond Forming Reactions of Chiral Cyclopent-2-enone and Cyclopentene-1-methanol, Both Spiroconnecting a 1,2:5,6-Di-O-isopropylidene-α-D-glucofuranosyl Ring.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
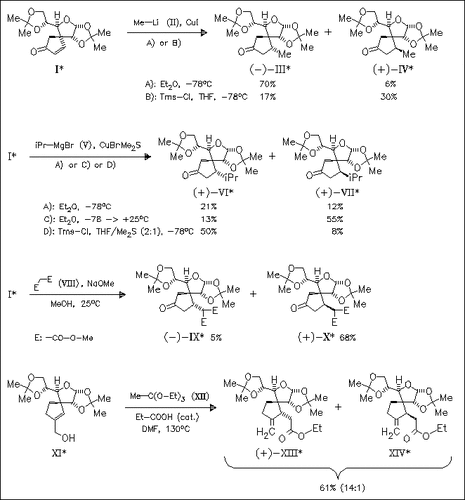
The conjugate addition reactions of various organocopper reagents [cf. (II) and (V)] as well as dimethyl malonate anion (VIII) to title cyclopentenone (I) are studied, and for most cases the optimal conditions are found, which provide the corresponding β-functionalized cyclopentanones in high to excellent diastereoselectivity. Additionally, the reaction of spirocyclic hydroxymethylcyclopentene (XI) with triethyl orthoacetate (XII) proceeds by a thermal Claisen rearrangement to provide spirocyclic methylenecyclopentylacetic acid ester (XIII) in high diastereoselectivity (87% d.e.).