ChemInform Abstract: 1,3-Dipolar Cycloaddition of Furfuryl Nitrones with Acrylates. A Convenient Approach to Protected 4-Hydroxypyroglutamic Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
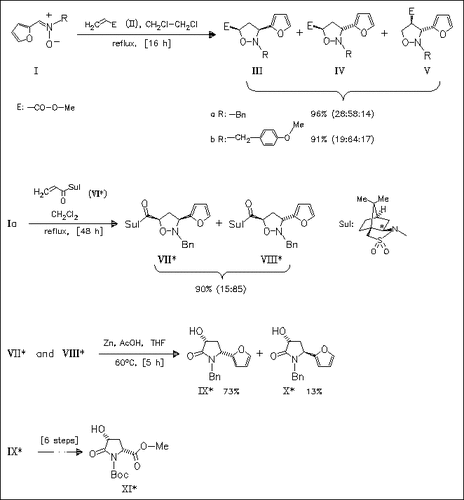
Furylnitrones (I) react with acrylates (II) to provide isoxazolidines (III), (IV), and (V) with good regio- and diastereoselectivity. The optically active acrylamide (VI) gives chiral cycloadducts (VII) and (VIII) which can be readily converted to optically pure pyrrolidin-2-ones (IX) and (X) as precursors of 4-hydroxypyroglutamic acids [cf. (XI)].