ChemInform Abstract: Two Comparisons of the Performance of Positional Scanning and Deletion Synthesis for the Identification of Active Constituents in Mixture Combinatorial Libraries.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
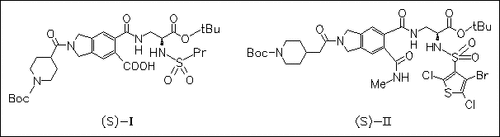
Vitronectin receptor binding inhibition is evaluated with two small libraries of 120 compounds each, which function as Arg-Gly-Asp mimics. Comparison of the performance of positional scanning and deletion synthesis deconvolution libraries to identify active constituents in mixture combinatorial libraries shows that the former is capable of identifying multiple hits at the expense of accurately identifying the most potent library members, i.e. compound (I) for library I and compound (II) for library II, whereas the latter one is more sensitive to identifying the most potent hits (mentioned above) but at the expense of differentiating weaker activities.