ChemInform Abstract: A Facile Access to 2-Methylthio/Alkoxy/Amino-3-acylimidazo[1,2-a]pyridines Based on Cupric Chloride Promoted Oxidative Ring Closure of α-Oxoketene N,S-, N,O-, and N,N-Acetals.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
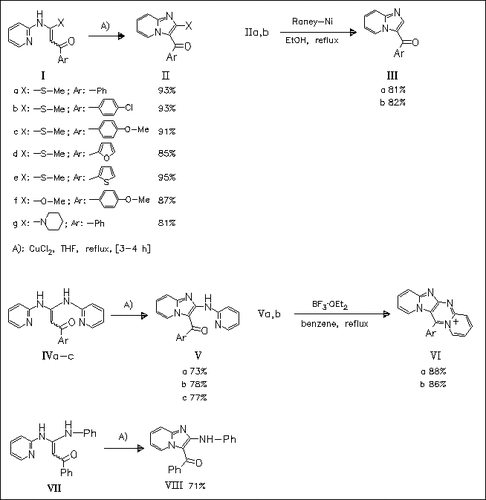
α-Oxoketene N,S- N,O-, and N,N acetals, easily accessible from aminoheterocycles, undergo under mild conditions in the presence of CuCl2 an unprecedented cyclization to give 2-methylthio-, 2-methoxy-, and 2-aminoimidazopyridines in good yields. A few of these compounds are further subjected to reductive dethiomethylation or cyclodehydration to give products (III) and (VI) respectively.