ChemInform Abstract: Synthesis of Optically Active 5-Substituted-2-pyrrolidinone Derivatives Having Atropisomeric Structure and 3,5-cis-Selective Reaction of Their Enolates with Electrophiles.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
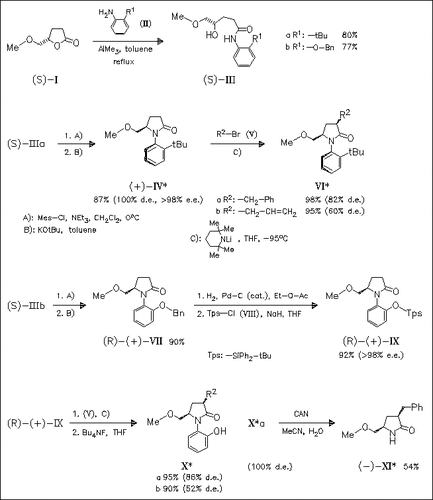
Starting from the butyrolactone (I) the pyrrolidinone (IV) having atropisomerism and the derivative (IX) having an atropisomerism-like structure can be prepared in optically pure form. The reaction of their Li-enolates with electrophiles allows stereoselective formation of 3,5-cis-substituted products, which is unusual.