ChemInform Abstract: Preparation, X-Ray Crystal Structures, and Reactivity of Alkynylcyclopropenylium Salts.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
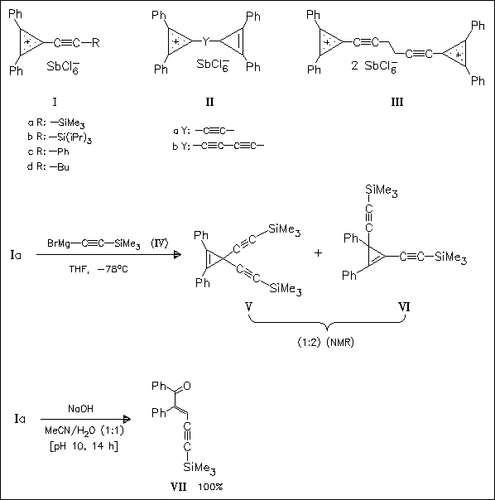
Several 3-alkynylcyclopropenes are synthesized and subsequently converted into alkynylcyclopropenylium salts (I) by hydride abstraction with the triphenylmethylium cation. The analogous conversion of ethyne- or butadiyne-bridged biscyclopropenes yields only the corresponding monocations (II). Preparation of a dication is only possible when an ethylene spacer is inserted between the acetylene groups as in (III). The structures of (Ia), (Ib), and (Ic) are determined by single-crystal X-ray analyses. Reaction of (I) with nucleophiles under kinetic control leads to a mixture of products, e.g. (V) and (VI), whereas under thermodynamic conditions nucleophilic addition is followed by ring opening to produce only one of three possible products, i.e. (VII). Further studies of the reactivity of 3-alkynylcyclopropenes are in progress.