ChemInform Abstract: Bidentate Chelation-Controlled Asymmetric Synthesis of α-Hydroxy Esters Based on the Glycolate Enolate Alkylation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
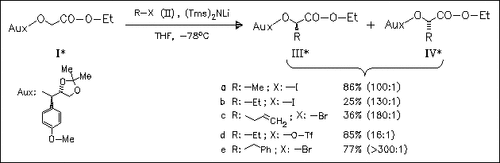
A new chiral auxiliary is developed and used for the asymmetric synthesis of optically active α-hydroxy esters (III). Mild reaction conditions, high stereoselectivity and the convenience of preparation and removal of the auxiliary make this method attractively.