ChemInform Abstract: An Investigation of Imidazole and Oxazole Syntheses Using Aryl-Substituted TosMIC Reagents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
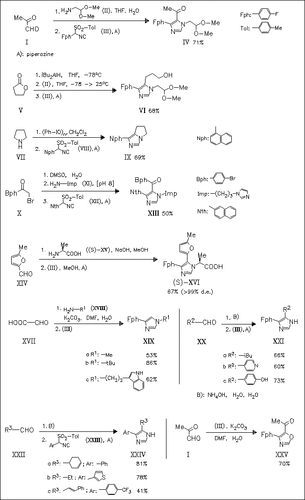
Aryl-substituted tosylmethyl isocyanides readily undergo cycloadditions with a variety of imines generated in situ to give 1,4,5-trisubstituted as well as 1,4- and 4,5-disubstituted imidazoles in one pot. The method can easily accommodate chiral amines and aldehydes to generate products with excellent retention of chiral purity. The cycloaddition of the aryl-substituted tosylmethyl isocyanides with aldehydes generates smoothly oxazoles.