ChemInform Abstract: Effective Ring-Opening Reaction of Aziridines with Trimethylsilyl Compounds: A Facile Access to β-Amino Acids and 1,2-Diamine Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
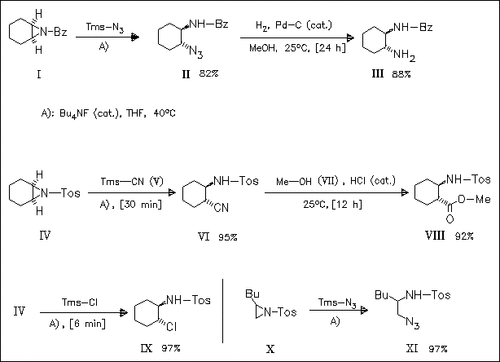
In the presence of catalytic tetrabutylammonium fluoride, aziridines undergo regioselective ring-opening with trimethylsilyl compounds to yield azido-, cyano-, or chloroamines. The products can be transformed to derivatives [cf. (III)] of vicinal diamines or β-amino acid esters [cf. (VIII)].