ChemInform Abstract: Cyano-Bridged Re6Q8 (Q: S, Se) Cluster-Cobalt(II) Framework Materials: Versatile Solid Chemical Sensors.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
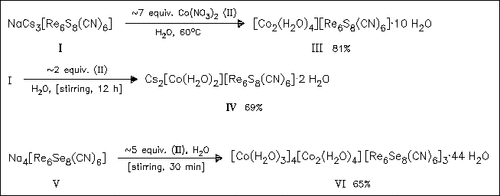
The structures of the compounds (III) (space group P21/n, Z = 2) and (VI) (C2/c, 4) are determined by single crystal XRD and that of (IV) (Imma, 4) by powder XRD. The structure of (III) consists of an extended Prussian Blue type framework containing face-capped octahedral [Re6S8]2+ clusters and [Co2(μ-OH2)2]4+ cluster cores enclosing water-filled cavities ≈258 Å in volume. The structure of (VI) consists of a network of Co2+ and [Co2(μ-OH2)2]4+ ions connected through [Re6Se8(CN)6]4- clusters, defining channels with minimum internal diameters of 4.8 Å. Upon exposure to Et2O vapor the color of (III) and (VI) immediately changes from orange to an intense blue-violet or blue; other polar solvents induce different colors. It is proposed that the vapochromic response is due to solvent molecules entering the pores of the solid and disrupting the hydrogen-bonded water network. Release of bound water from the [Co2(μ-OH2)2]4+ clusters results in a change of the Co coordination sphere from octahedral to tetrahedral. Spectroscopic and magnetic measurements confirm the change in coordination geometry and the trends in solvent response (MeOH < EtOH < PrOH < iPrOH) are consistent with a reduced ability to support the bridging water ligands of the clusters. Size-selective sensing is demonstrated with tert.-butyl ether, which causes a color change in (VI), but not in (III). Compound (IV) displays no vapochromic effect.