ChemInform Abstract: Efficient Synthesis of S-Adenosyl-L-Homocysteine Natural Product Analogues and Their Use to Elucidate the Structural Determinant for Cofactor Binding of the DNA Methyltransferase M×HhaI.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
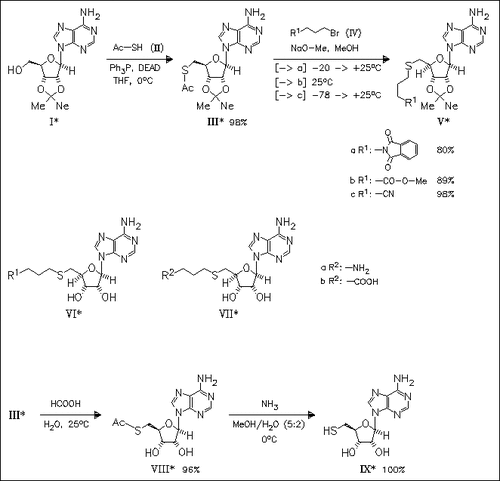
Key step of the synthesis of S-adenosyl-L-homocysteine analogues is the direct reaction of isopropylideneadenosine (I) with thioacetic acid under Mitsunobu conditions to furnish 5′-acetylthio derivative (III) without the need to protect the free amino group. Alkylation of compound (III) with various alkyl bromides (IV), followed by acetonide deprotection to derivatives (VI) and, for derivatives (VIa), (VIb), hydrolysis of the terminal functional groups, provides access to title adenosylhomocysteine analogues modified in the amino acid moiety (VIc) and (VII). Finally, 5′-thioadenosine (IX) is obtained in excellent yield from compound (III) by only two deprotection steps [overall yield from O,O-diprotected adenosine (I) 95%].