ChemInform Abstract: The Efficient Entry into the Tricyclic Core of Halichlorine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
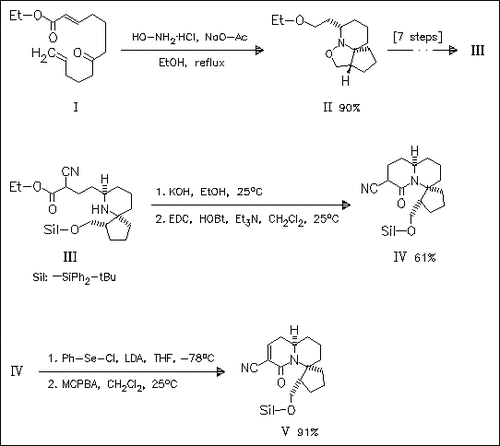
The synthesis of the tricyclic core (V) of halichlorine starts from Grigg′s tricyclic isoxazolidine (II), which is easily available by tandem intramolecular Michael addition—[3 + 2] cycloaddition of the oxime of (I).